New organic ferroelectric materials
It would be highly desirable to find a new class of inexpensive easily produced ferroelectrics that do not require the high temperature and slow processing required by oxide synthesis and crystal growth. The most used ferroelectrics are perovskites, which need high-temperature synthesis and the most commonly used, Pb(Zr,Ti)O3 (PZT), contains environmentally poisonous metals. Organic ferroelectrics would be attractive alternatives for many applications, because they could be nontoxic, flexible, and easy to process.
In contrast with perovskite ferroelectrics, which are polar from displacements of ions, organic ferroelectrics typically function through reorientation of polar molecules or hopping of hydrogen bonded protons. In inorganic perovskite ferroelectrics, large electromechanical response arises from polarization rotation. Can such a system be designed in organic ferroelectric material? Experiments on MBI (C9H8N2) show robust polarization of 5 μC/cm2 and ferroelectricity at room temperature. Although that polarization is large for organics, oxides such PZT have polarizations of 30-100 μC/cm2. One of our goals will be to design new organic ferroelectrics with larger polarizations through crystal structure searching, but much can be said to understand better experimentally realizable systems and understand how best to optimize them.
Can polarization rotation be used to obtain large electromechanical response? Although the polarizations are low, they are also much softer than oxides, so significant actuation can still be obtained, and perhaps they could also be used as transducers. We will study the energetics and polarization as a function of applied field magnitude and direction using DFT with vDW functionals, which we have found to be reasonably accurate for molecular systems such as benzene.
Simulate and tune ferroelectric materials for electrocaloric application
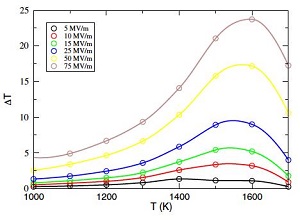 The electrocaloric effect (ECE) can be used for solid state refrigeration, and is inherently much more efficient than conventional gas cycle refrigeration; it can in principle be developed for use in thin film form to allow on chip active cooling, having tremendous potential for high speed computing. The ECE arises from the change in entropy with electric field, which is equivalent throgh a Maxwell relation to the change in polarization with temperature at constant field.
The electrocaloric effect (ECE) can be used for solid state refrigeration, and is inherently much more efficient than conventional gas cycle refrigeration; it can in principle be developed for use in thin film form to allow on chip active cooling, having tremendous potential for high speed computing. The ECE arises from the change in entropy with electric field, which is equivalent throgh a Maxwell relation to the change in polarization with temperature at constant field.
Using MD with a first-principles based shell-model, we showed that the ECE is highest around the ferroelectric phase transition, and can even be higher in the paraelectric field above the phase transition, or in non-ferroelectrics like PMN.
Figure. Change in temperature with applied field and temperature from the ECE computed for LiNbO3 from shell-model MD. The large changes in temperature can be used in an efficient refrigeration cycle, but the effect becomes much smaller at room temperature. Thus ones needs materials with much lower Tc’s.
In the Rose and Cohen paper, we studied LiNbO3 , which has a very high Tc. Here we will study materials with Tc below room temperature to find optimal materials for room temperature or lower refrigeration. Recently it has been shown that a negative ECE can be found in special cases, that is, materials cool when an electric field is applied, whereas they warm when the field is withdrawn, the opposite of the normal behavior. This is counter intuitive, because the conventional picture of the ECE is that of dipoles oriented with applied electric field, lowering the entropy. In the negative ECE, the entropy must increase with applied field, and such behavior is understood to arise from electric field driven phase transitions. With further increase in temperature, these materials become normal again. The conjunction of the negative and positive ECE potentially doubles the efficiency of solid state ECE refrigeration.
We will study PMN-xPT as described above, to find the optimal composition and conditions for the ECE in these high coupling materials materials. We will also plot the phase diagram with respect to external electric field, composition and temperature.
P2 symmetry in MnWO4
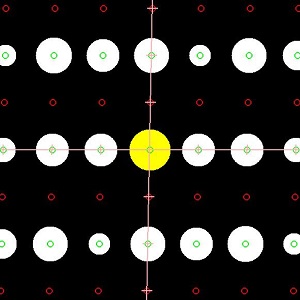 Multiferroic materials, in which ferroelectric polarization can be driven by the magnetic ordering and vice versa, have become a subject of much attention for their magnetoelectric effects. Many of these single-phase materials possess geometrically frustrated spin networks, which generally prevent the formation of conventional collinear spin structures. In this class of antiferromagnetic materials, an incommensurate (ICM) magnetic structure with spiral-spin order and the magnetic phase transition to this noncentrosymetrically ordered magnetic state can induce a spontaneous electric polarization via the spin-orbit coupling.
Multiferroic materials, in which ferroelectric polarization can be driven by the magnetic ordering and vice versa, have become a subject of much attention for their magnetoelectric effects. Many of these single-phase materials possess geometrically frustrated spin networks, which generally prevent the formation of conventional collinear spin structures. In this class of antiferromagnetic materials, an incommensurate (ICM) magnetic structure with spiral-spin order and the magnetic phase transition to this noncentrosymetrically ordered magnetic state can induce a spontaneous electric polarization via the spin-orbit coupling.
Mineral huebnerite, manganese tungstate (MnWO4), has long been considered as the paramagnetic(PM) and paraelectric(PE) with a centrosymmetric space group P2/c at room temperature. Very recently, Prof. S.H. Park’s group in LMU of Munich observed a new polar symmetry of this material with ferrodistrortive domains (J. Magn. Magn. Mater. 394(2015)160–172). In their experiments, large-size single-crystal samples of huebnerite natural multiferroic MnWO4 were analyzed by neutron and synchrotron X-ray single-crystal diffraction as well as by polarized Raman spectroscopy. Both neutron and X-ray diffraction analyzes reveal polar space-group symmetry P2 for the nuclear structure of MnWO4 via the detection of weak reflections h0l forbidden for the gliding plane c. Micro X-ray diffraction and Raman-scattering mapping reveal a ferrodistortive domain texture in the room-temperature paramagnetic state of MnWO4.
Cooperating with Prof. Park, we are conducting density functional theory (DFT) +U calculations of the MnWO4. In our preliminary analysis, the forbidden reflections for P2/c are extremely sensitive to the O displacement in the Gamma1 - A2 symmetry distortion. We will prove the forbidden reflections are mainly caused by an octahedral rotation which breaks the glide plane, which is more like an improper ferroelectric in perovskites.

